What is Atom? Definition, Types, Examples and Facts
Hello, how are you guys hope you are very fine and reading our articles on the daily basis. we are presenting today a most important topic of Physics what is an atom? you are all the people listening to atoms but do you know what is atom definition or what is atoms in science? atom is also called the building block of any substance. The thing which we can feel and touch is everything is made up of the atom. We will give you a deep knowledge of atoms in this article so that you are able to answer the typical questions related to the atoms asked in any competitive exams.
There are various types of questions asked in many subjective types of exams and also competitive exams. These questions may be like what does atom mean, what is the atom made of, what is an atom, what is the atom, Facts about Dalton’s Atomic Theory, atom vs molecule, etc. So guys you must read our article from the starting paragraph to the ending paragraph. For reading our previous articles on the Physics subject you can go through the link given below. So without wasting any time let us start our today’s topic what is atom?
What is Atom (Definition)?
The atom is the smallest and the fundamental unit of any matter which contributes to making the whole substance. The atom possesses all the chemical properties of an element. the bun important thing about the atom is that it cannot exist independently, which means a single atom does not exist in the universe, Some exceptions may be present such as helium, neon, and noble gases atoms. Two or more atoms combine and constitute molecules. Many molecules combined together and further made the building blocks of any matter. The great scientist Dalton very first describe atoms.
Atoms are very very small units that we cannot see with the help of advanced microscopes also. Many scientists give the hypothesis of the structure of the atoms. Many great scientists such as Rutherford, Neels Bohr, etc contribute to give the structure of atoms.
Constituents of an Atom
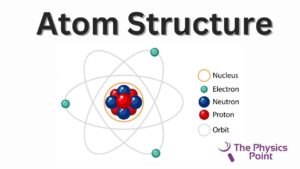
Many of you guys thinking about what is the atom made of (What is Atom), Atom is also made up of some particles. The atom has two parts first one is the nucleus and the other one is the electrons. Inside the nucleus of an atom, there are two other particles are called neutrons and protons. there are some other also particles that exist in the nucleus that is quark discovered recently. The atom is overall neutral. the nucleus possesses a positive charge due to the charge of the protons. inside the nucleus, Proton binds with the neutron by the nuclear binding force.
Neutrons are neutral particles and possess no charge but Proton has a positive charge, that’s why the nucleus of the atom has a positive charge. To counteract this positive charge, outside of the nucleus there exist electrons that have negative charges. both proton and electrons neutralize the atoms. Proton neutrons and electrons are called atom particles. Protons and neutrons in the combined form are called nucleons.
One thought that you are facing is what is the difference between an atom and a molecule so we told you that the difference between atom and molecule is that an atom is a single unit and the molecule consists of many atoms.
Example of Atoms
There are approx 118 kinds of atoms or elements discovered now. Examples of some atoms like Hydrogen, Helium, Lithium, beryllium, Boron, carbon, neon, iron, calcium, magnesium, aluminum, etc These 118 kinds of atoms are put in the modern periodic table according to their properties.
Facts about Dalton’s Atomic Theory
- Very first it was only Dalton who said that any matter is composed of many many particles known as atoms.
- They also said that atoms are indestructible particles or indivisible particles and they cannot be created or destroyed by any method in the universe.
- Different types of atoms possess different types of chemical properties whereas the same type of atoms possess the same types of chemical properties.
- Atoms combined with other atoms to form a molecule and the ratio of combination is a small whole number.
Thomson Atomic Model
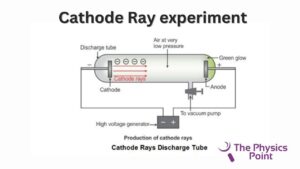
Scientist Sir Joseph John Thomson also give the model of atoms in the time of 1900s. This was the fourth model of the atom given by any scientist. he also discover the electrons and for the doing discovery of electrons, he was awarded the Nobel Prize. By performing the Cathode Ray experiment, he discovered electrons.
Types of Atoms
Atoms are divided into many types on the basis of different types of criteria. Here we divide atoms into 5 different types on the basis of stability, mass number, ionization, and radioactivity.

1. Stable Atoms
These are the atoms that are chemically non-reactive in nearly all conditions. such as some noble gases.
2. Isotopes
These are atoms that have the same number of protons but different numbers of neutrons. The chemical properties of these types of atoms are the same but they are different in physical properties and also atomic mass.
3. Radioactive Atoms
The atoms which are radiating are known as radioactive atoms. Because many neutrons in the nucleus of these types of atoms make them unstable, they start to emit the particles until they become stable.
4. Ions
The atoms which possess extra electrons or lose their electron are known as ions. They possess some net charge.
5. Antimatter
Every atomic particle has its twin antimatter by having an opposite charge. These types of matter are very rarely covered and fragile.
Conclusion
In today’s topic what is atom we told you everything in depth about atoms. The discovery of an atom is a very useful discovery in science. Every year many important questions are asked about this topic in every type of exam. if you have any doubts related to this topic (What is Atom) or any suggestions you can contact us through the comment section box. Very soon we will again meet with the new article on Physics.
