What is Second Law of Thermodynamics? Statement, Examples, Equation
Hello guys, how are you? hope you all are very fine and enjoying Our article on the daily basis. In this article, we will tell you about the second law of thermodynamics. the second law of thermodynamics states that heat cannot transfer from a low body to a high body without any work. thermodynamics branch Is a very big branch and dominant branch of Physics so read this article very carefully so that no concept will be missed from your understanding. You can also read our previous article on thermodynamics on our website.
Some very important questions from the exam point of view will be described in our article today and these questions are like the second law of thermodynamics examples, the second law of thermodynamics definition, the second law of thermodynamics equation, the second law of thermodynamics simple, which of the following statements is a logical consequence of the second law of thermodynamics, etc. So without wasting much time rates move on our today the article.
What is the second law of thermodynamics?
The second law of thermodynamics states that in the universe if there is any process happening by itself means spontaneously that it will always lead to the increase in entropy of the system or the universe. the second law of thermodynamics states that the system’s entropy always goes on increasing which is isolated.
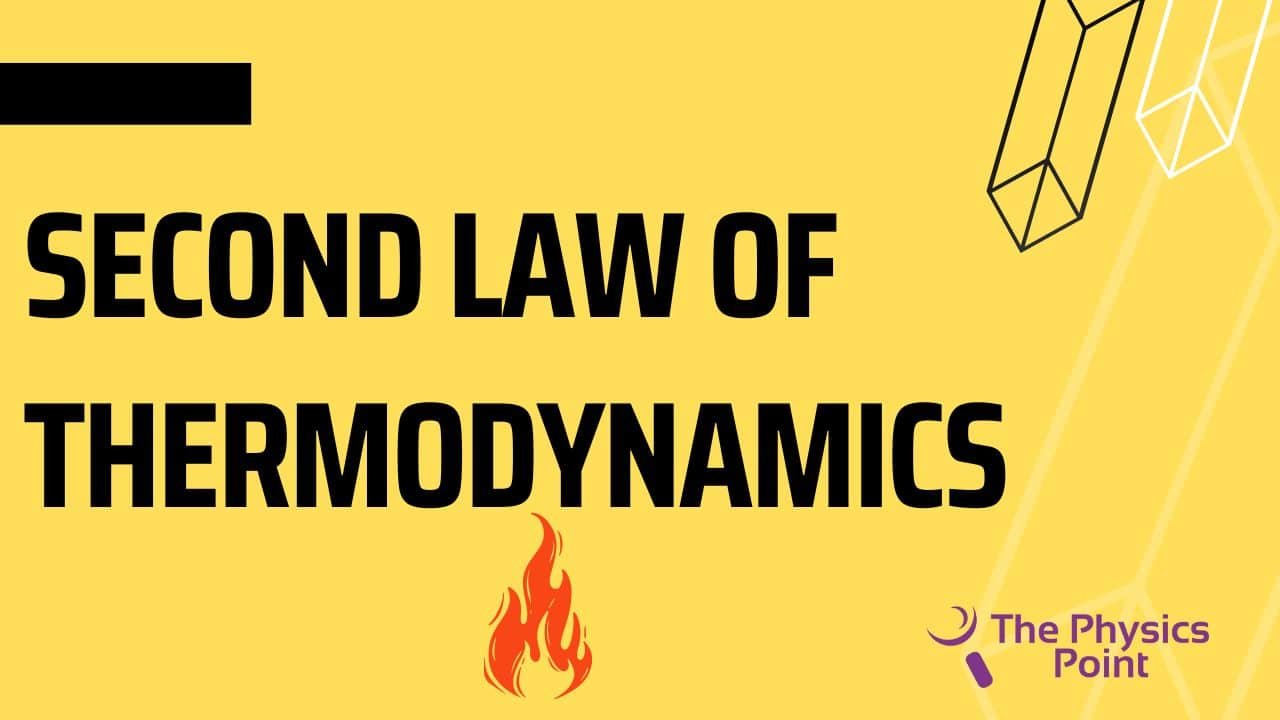
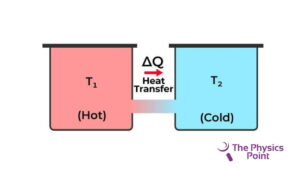
The second law of thermodynamics restricts the direction of heat from the low temperature to the high temperature. they need the second law of thermodynamics because the first law of thermodynamics fails to give information about the direction of the energy transfer or the heat flow. The first law only says about the quantity of the transfer. The second law explains it clearly that it is not possible to exchange heat energy for mechanical energy with an efficiency of 100%. because to convert the energy into mechanical energy we need to do some work in the form of heat loss.
The second law of thermodynamics equation
Now we are going to represent the equation of the second law as given below
ΔSuniv > 0
here ΔSuniv indicates the change in the entropy. Here we consider the entropy of the universe.
The randomness of any system is measured in the form of Entropy or we can also say that the measure of energy or chaos of an isolated system is considered entropy. it can also be considered as an index of quantity that defines the quality of energy. The increase in the entropy of the system can be understood in the following two ways –
- For a closed system, the mass is not going to be exchanged with the surrounding which means the mass remains constant. But there is an exchange of heat within the surrounding and this exchange of heat makes a disturbance in the whole system and therefore results in the increase in the entropy of that system.
- In another way, internal changes make the systems molecule in the movement and this movement creates a disturbance in the system. This disturbance is Irreversible inside that system and results in the increment of the entropy of the system.
Kelvin plank statement
According to the Kelvin plank statement, it is not possible for a heat engine to produce a network or the connection in a complete cycle if the exchange of heat is happening only within the two bodies at a constant temperature. but the heat engine is the exception to the Kelvin plank statement because the heat engine can produce work only in one complete cycle only one reservoir. Thus we can say that the heat engine does not follow the Kelvin plan statement.
Clausius’s statement
According to Clausius’s statement, there is not any device discovered at the current time that can transfer heat from a body of cold temperature to a body of hot temperature without consuming any type of work. Energy cannot flow by itself from cold temperature to hot temperature. The heat pump and refrigerator work according to Clausius’s statement.
Some solved examples of the second law of thermodynamics:-
Example-1. A heat pump uses 400 J of work to remove 500 J of heat energy from the low-temperature reservoir. How much heat is transferred to a higher-temperature reservoir?
Soln:
W = 400 J
QC = 500 J
QH = W + QC
QH = 400 J + 500 J
QH = 900 J
The Heat transferred to the higher temperature source or reservoir is 900 Jouls.
Example-2. Refrigerators and heat pumps work on the principle of the second law of thermodynamics. According to the second law of thermodynamics you have to do some work if you want to transfer the heat energy from the lower temperature to the higher temperature. Also in the reverse Carnot cycle, we use the work to move heat from a body of lower temperature to a body of higher temperature and the original Carnot engine uses heat for the production of work.
Frequently asked questions (FAQs)
Ques. What is the second law of thermodynamics?
Ans. The second law of thermodynamics states that in the universe if there is any process happening by itself that it will always lead to an increase in entropy in the universe.
Ques. Which of the following statements is a logical consequence of the second law of thermodynamics?
Ans. According to the second law, every type of chemical reaction must lead to an increase in the total entropy of the universe.
Ques. What is Clausius’s statement regarding the second law of thermodynamics?
Ans. According to Clausius’s statement, there is not any device discovered at the current time that can transfer heat from a body of cold temperature to a body of hot temperature without consuming any type of work.
Conclusion
In today’s article we have learned many things about the second law of Thermodynamics and some important questions were described in our article to make your concepts very clear. These questions are like the Second Law of Thermodynamics, thermodynamics second law, 2nd law of thermodynamics, what is the second law of thermodynamics, the second law of thermodynamics states that. If you have any doubts or any questions related to this article you can comment in the comment section box. We will meet in the next article on physics very soon.
