Zeroth Law of Thermodynamics | Definition, Example and Application

Hello, how are you, people? You are very welcome to our website. We are here to present a new article on the Physics zeroth law of thermodynamics. This is one of the important laws of the four laws of Thermodynamics and you must know about this due to its popularity. Before it from our website you can read thermodynamic processes and thermodynamics in every detail. for your information we tell you this law of thermodynamics was discovered many times after the three laws of thermodynamics. Thermodynamics is a big branch of Physics that has many processes and its own terminology so to understand it very well please read the whole article from start to end.
Important questions of today’s article such as thermodynamics zeroth law, 0th law of thermodynamics, the zeroth law, what is zeroth law of thermodynamics, what is the zeroth law of thermodynamics, zeroth law of thermodynamics example, zeroth law of thermodynamics definition, what is the 0th law of thermodynamics, zeroth law of thermodynamics equation, it is here constantly asked in different types of examination so you have to learn these questions from our website as soon as possible. so without wasting much time let us start our today’s article.
What is the zeroth law of thermodynamics?
This is one of the fundamental laws of the four laws of thermodynamics. The credit for the zeroth law of thermodynamics goes to the scientist Ralph H. Fowler. This law was discovered much later after the original three laws. According to the law, when there is a body named a is in thermal equilibrium with another body called B and also separates in thermal equilibrium with another body C then it is observed that body B and the body sea will also be in the thermal stability or in equilibrium with each other. This law of thermodynamics is totally based on temperature measurement.
You can understand this in another way, according to the zeroth of thermodynamics if there are two systems that are in thermal equilibrium with each other and also with the third one then the three systems are also observed in thermal equilibrium of bodies with one another. So one thing is observed if systems are in a thermal equilibrium state then they are maintained at the same temperature.
What is thermal Equilibrium?
Temperature is one of the properties of Physics that can distinguish two bodies in the hot and cold criteria. Thermal equilibrium can be defined as two bodies or more bodies are maintained at different temperatures and brought closer in contact, then after some time all bodies obtain a common temperature and all bodies (systems) are called to exist in thermal equilibrium. We can also define thermal equilibrium as if there is no exchange between the systems then they are in the thermal equilibrium.
Let us understand this thermal equilibrium with an example, if they put some food in the refrigerator for the whole night then the observation is that the food is in the thermal equilibrium with the temperature of the refrigerator and there is no flow of heat from the food to the refrigerator or the refrigerator to the food this state is called as thermal equilibrium.
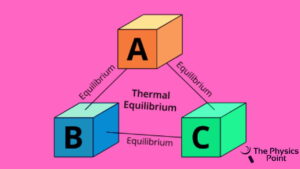
Zeroth law of thermodynamics example and applications
This law is mostly used in the comparison of the body temperature and all the system temperatures. and it is a very important law of thermodynamics for mathematical calculations. If we want to measure the correct temperature of any body, we require a reference body and from here we measure the change in temperature due to certain characteristics of that body, and these characteristics are called thermodynamic properties.
The most common application of this law of thermodynamics can be observed in the thermometers and the very most common thermometer for the observation of zeroth of thermodynamics is the mercury thermometer.

Some Frequently Asked Questions (FAQs)
Ques. Define thermal equilibrium
Ans. Thermal equilibrium can be defined as two or more bodies being maintained at different temperatures and brought closer in contact, then after some time all bodies attain a common temperature and all bodies (systems) are called to exist in thermal equilibrium.
Ques. What is the zeroth of thermodynamics?
Ans. According to the zeroth of thermodynamics if there are two systems that are in thermal equilibrium with each other and also with the third one then the three systems are also in thermal equilibrium with one another.
Ques. What is the meaning of internal energy?
Ans. The internal energy of the system can be defined as the sum of total energy inside a system such as kinetic energy and potential energy.
Ques. What is the meaning of temperature?
Ans. Temperature is the physical quantity that tells about the coldness or hotness of the body or any system. We can also state that temperature is a physical quantity that tells about the thermal equilibrium, whether the system with the other system is in equilibrium in contact or not.
Conclusion
The final word of the site that we have learned many things about the zeroth law of thermodynamics such as some important questions about the 0th law of thermodynamics, what’s the zeroth law of thermodynamics, define the zeroth law of thermodynamics, the application of zeroth law of thermodynamics, etc. Still, if you have any confusion or any doubt you can contact us below the article with the help of the comment section works we will try to give your reply as soon as possible.
