What is Coulomb’s Law? Definition, Formula, Equation, Examples
`How are you guys once again you are very welcome to our physics website. we are presenting another more interesting topic of electrostatics- What is Coulomb’s Law? in the branch of electrostatics the study of the property of charges and their behavior and The interaction with other charged bodies. Coulomb’s Law Describe the force of repulsion or attraction between the two charge bodies. We will discuss What is Coulomb’s Law in today’s article in very simple words. Every concept of Coulomb’s law will be included in this important article – What is Coulomb’s Law?
From the exam point of view this topic, What is Coulomb’s Law is very important. Every physics student must know about this some repeatedly ask questions like coulomb’s law equation, coulomb’s constant, k constant physics, what is k in coulomb’s law, coulomb’s law example, What is Coulomb’s Law expression, etc. These all questions will describe in our today’s article. You can also read our previous article on our website which will increase your knowledge of Physics.
Coulomb’s Law (Definition)
Coulomb’s law on the force of attraction or repulsion between two body that possesses some charge. According to this law, The attraction force or the repulsion force acting between two charged particles is directly proportional to the product of the magnitude of their charges and in the inverse proportion of the square of the distance between the charges. This coulomb force act at the line of joining of two charges and consider the charges as point charges.
Now we are going to talk about the history of Coulomb’s law, a great physicist of French – Charles Augustine De Coulomb, In 1785 will give a mathematical relationship between two bodies that possess some charge. He stated that there is a force of repulsion or attraction between two charged bodies. This statement of Coulomb is now known as Coulomb’s law or Coulomb’s inverse-square Law. This law (What is Coulomb’s Law) also follows the theorem of superposition.
Coulomb’s Law Formula
According to the law of Coulomb, the coulomb’s law derivation & formula of Coulomb are given below –
F ∝ q1q2
And F ∝ 1/r2
F = k q1q2/ r2
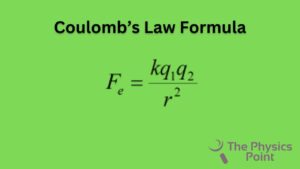
where,
- ε means absolute permittivity,
- K or εr indicates the relative permittivity or specific inductive capacity.
- ε0 represents the permittivity of free space. The value of ε0 is 8.854 × 10-12 C2 N-1 m-2
- K or εr is also called a dielectric constant of the medium (in which both charges are kept) and the k value in physics is 9 × 109 Nm2/ C2
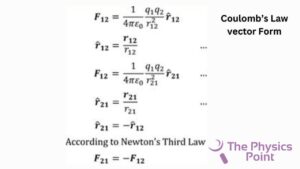
The vector form of this electrostatic Coulomb’s law is given below as –
Restrictions of Coulomb’s law
It has some restrictions or it can be said that this law has some limitations. These limitations are stated given below in very simple words –
- Coulomb law is only applicable to the point charges only which are at the rest.
- Coulomb’s Law is only applied in the situation where the inverse-square law is obeyed strictly.
- The charged body must not be in an arbitrary shape (random), because in this condition we are unable to determine the separation between the charges.
- This law is not applicable to calculate the force of attraction or repulsion on big celestial bodies like planets and satellites.
Application of Coulomb’s law
Coulomb’s law is the only law in Physics to determine the force of attraction or repulsion between two small charge bodies. Its application is given below-
- We can calculate the separation means distance and force acting between the two charges.
- The most important factor of electrostatic is the electric field is also calculated using this Coulomb law. the relation between the coulombs force and the electric field is given as
F = Q E
Here,
- E indicates the electric field strength.
- F indicates the electrostatic force.
- Q means for the charge in coulombs (SI unit).
Some important facts about Coulomb’s law
- The force acting between two charges in different mediums is the same but the separation between the charges is different than
Kr2 = constant or K1r12 = K2r22
- In a vacuum, two charges are separated by a distance r and the other two charges are placed in a medium of medium constant k with the separation of r0 between them, then for the same coulomb force
Kr2 = r02
- The ratio of electrostatic force and the gravitational force between two electrons separated by a distance r is 1042
- The ratio of electrostatic force and the gravitational force between a proton and an electron separated by a distance r is 1039
- The velocity of light (c) the permittivity of free space and the permeability of free space, these three things that have a relationship shown as c = 1 / √ (μoεo )
Examples of Coulomb’s law
Mixing anything in the water
When we dissolve something in the water, the polarity of the substance and water play a major role in the solubility of that substance. You can observe this at your home. Sometimes you deserve salt or table salt in the water, you see that table salt dissolves very finely in water. This is because of the polarity nature of sodium chloride (salt), which attracts the water molecules and get dissolves completely.
Charged Rod
When you take a glass rod and rub it on your hair or any piece of silk cloth, you observe that the glass rod attracts pieces of paper and other small and light things. By the action of rubbing the rod gets charged and it is able to attract or repeal things according to the electrostatics.
The Conclusion
We have discussed many things about What is Coulomb’s Law, coulomb’s force, the coulomb’s law formula, and the coulomb’s law equation. If you found this article helpful and knowledgeable to you please share this with your friends also so that they also get benefit from our website. For any query or suggestion related to the article What is Coulomb’s Law, you can contact us through the comment section we will try to very soon. Thanks!
